Ozone Layer Depletion is a global environmental issue that has captured the attention of scientists and policymakers alike. The ozone layer, vital for protecting life on Earth, is being compromised due to human activity. This depletion allows harmful ultraviolet (UV) radiation to reach the Earth’s surface, posing serious risks to human health, ecosystems, and the environment. In this blog, we will delve deep into the science of ozone depletion, its causes, its wide-reaching effects, and what is being done to address it.
Table of Contents
Understanding Ozone Layer
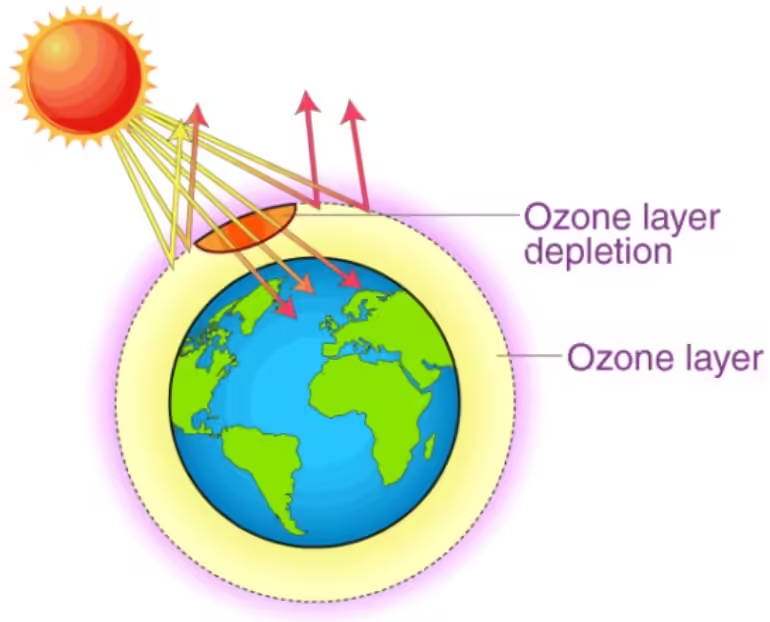
The ozone layer, located in the Earth’s stratosphere, comprises a high concentration of ozone (O₃) molecules. It acts as a protective shield by absorbing most of the Sun’s harmful UV-B radiation. However, the integrity of this layer is being threatened by various human-made chemicals, leading to what we know as ozone layer depletion.
Why Is Ozone Important?

The ozone layer serves as a crucial shield, protecting all forms of life on Earth from harmful ultraviolet radiation. Without it, the Sun’s harmful UV-B rays would penetrate the atmosphere, leading to a wide range of health problems for humans and damage to ecosystems. The breakdown of this layer, referred to as ozone layer depletion, can lead to an increase in UV radiation exposure, significantly affecting human health and environmental stability.
Causes of Ozone Layer Depletion

1. Chlorofluorocarbons (CFCs)
One of the primary culprits behind ozone depletion is the release of chlorofluorocarbons (CFCs). Used extensively in refrigeration, air conditioners, and aerosol sprays, CFCs release chlorine atoms when broken down by UV light in the stratosphere. These chlorine atoms actively destroy ozone molecules, leading to the thinning of the ozone layer.
2. Halons
Halons, primarily used in fire extinguishers, contain bromine, which is even more destructive to ozone than chlorine. Like CFCs, halons release bromine into the atmosphere, which accelerates layer depletion by breaking down ozone molecules.
3. Nitrous Oxide (N₂O)
Nitrous oxide, primarily produced through agricultural practices and the burning of fossil fuels, also contributes to ozone layer depletion. Nitrous oxide releases nitrogen oxides (NOx) when broken down in the stratosphere, which reacts with ozone and causes its depletion.
4. Other Ozone-Depleting Substances (ODS)
Other chemicals, including carbon tetrachloride and methyl chloroform, are also harmful contributors to ozone layer depletion, although they are now less common due to regulatory actions.
How Ozone Layer Depletion Happens
The process of layer depletion occurs through a series of chemical reactions involving ozone-depleting substances (ODS). The main steps include:
- Release of ODS: Chemicals like CFCs and halons are released from industrial activities.
- Rising to the Stratosphere: These chemicals gradually rise into the stratosphere, taking several years to reach the ozone layer.
- Breaking Down by UV Light: Once in the stratosphere, these chemicals are broken down by UV radiation, releasing chlorine or bromine atoms.
- Ozone Destruction: These atoms react with ozone molecules, breaking them apart and reducing the overall concentration of ozone.
Each chlorine atom can destroy thousands of ozone molecules, making the process of ozone depletion highly destructive and long-lasting.
The Effects of Ozone Layer Depletion
1. Health Impacts on Humans
The Effect of ozone layer depletion on human health is profound. With a thinner ozone layer, more UV-B radiation reaches the Earth’s surface, leading to increased risks of skin cancer, cataracts, and immune system suppression.
- Skin Cancer: Prolonged exposure to UV-B rays can damage DNA in skin cells, leading to various types of skin cancer, including melanoma.
- Cataracts: Excessive UV exposure can accelerate cataract formation, leading to impaired vision or blindness.
- Immune System Suppression: Increased UV-B exposure can weaken the immune system, making the body more susceptible to infections and diseases.
2. Environmental Effects
The Effect of ozone layer depletion extends far beyond human health, impacting ecosystems and biodiversity.
- Marine Life: Increased UV radiation can reduce plankton populations, which form the base of the marine food chain. This can disrupt entire ocean ecosystems, affecting fish populations and global fisheries.
- Agriculture: Crops, such as wheat, maize, and rice, are sensitive to UV radiation. Ozone Layer Depletion can hinder crop growth, reduce yields, and ultimately threaten food security.
- Wildlife: Species like amphibians are particularly vulnerable to UV-B radiation. Their reproductive systems and skin can be damaged, leading to declining populations and disturbing entire ecosystems.
How Ozone Layer Depletion Influences Climate Change
While ozone layer depletion and climate change are distinct issues, they are interconnected. Many substances that cause ozone layer depletion, like CFCs, are also potent greenhouse gases that contribute to global warming. Additionally, changes in the ozone layer can affect weather patterns and atmospheric circulation, indirectly influencing climate change.
Mitigating ozone layer depletion can also aid in reducing global warming, as the phased-out substances are no longer contributing to the greenhouse effect.
Efforts to Combat Ozone Layer Depletion: The Montreal Protocol
The most significant global effort to address ozone layer depletion is the Montreal Protocol, signed in 1987. This global agreement was crafted to systematically reduce and eventually eliminate the production and use of substances that harm the ozone layer.
Achievements of the Montreal Protocol:
- Phasing Out ODS: The treaty successfully reduced the use of CFCs, halons, and other harmful chemicals, with many countries complying by adopting alternative technologies.
- Ozone Layer Recovery: Recent studies indicate that the ozone layer is slowly recovering and could return to pre-1980 levels by the middle of this century, thanks to the reduction in ODS emissions.
The Future of the Ozone Layer
The ongoing recovery of the ozone layer shows that concerted global action can reverse environmental damage. However, ozone layer depletion is not entirely behind us. Continued vigilance is required to ensure that ODS is not reintroduced into the environment and that alternative chemicals are safe for the ozone layer.
How You Can Help Fight Ozone Layer Depletion

While much of the effort to combat ozone layer depletion is led by governments and industries, individuals can also make a difference:
- Avoid Products with ODS: Choose products that are labeled “ozone-friendly” and avoid old appliances that may contain CFCs.
- Support Sustainable Energy: Reduce your carbon footprint by using renewable energy sources and energy-efficient appliances. This can also help reduce the substances that contribute to ozone depletion and climate change.
- Spread Awareness: Educate others about the importance of protecting the ozone layer and the long-term consequences of ozone depletion.
A Path to a Healthier Planet
Ozone Layer Depletion has far-reaching consequences, affecting human health, the environment, and global ecosystems. However, thanks to international agreements like the Montreal Protocol, progress is being made in protecting this vital part of our atmosphere. By understanding the causes and effects of ozone layer depletion, we can all contribute to its recovery and ensure a safer, healthier planet for future generations.
Frequently Asked Questions (FAQ)
1. What is Ozone Layer Depletion?
Ans: Ozone Layer Depletion occurs when the Earth’s stratospheric ozone layer thins out, reducing its ability to shield us from harmful UV radiation. This thinning allows more ultraviolet (UV) rays, especially UV-B, to penetrate the atmosphere, which poses serious risks to human health, ecosystems, and the environment.
2. What factors contribute to Ozone Layer Depletion?
Ans: The primary causes of ozone layer depletion are human-made chemicals known as ozone-depleting substances (ODS). These include:
- Chlorofluorocarbons (CFCs), used in refrigerants and aerosol sprays
- Halons, found in fire extinguishers
- Nitrous Oxide (N₂O), mainly from agricultural activities
- Other chemicals like carbon tetrachloride and methyl chloroform
These chemicals, when exposed to ultraviolet light in the stratosphere, release chlorine and bromine atoms, which then react with and deplete ozone molecules.
3. What is being done to stop Ozone Layer Depletion?
Ans: The most effective international effort to combat ozone layer depletion is the Montreal Protocol, signed in 1987. This global treaty aims to phase out the production and consumption of ozone-depleting substances (ODS). The protocol has been successful in reducing the emission of harmful chemicals, and recent studies show that the ozone layer is slowly recovering.
4. Is the ozone layer recovering?
Ans: Yes, thanks to the efforts of the Montreal Protocol, the ozone layer is gradually recovering. It is expected that the ozone layer could return to pre-1980 levels by the middle of this century, assuming continued global compliance with the treaty.
5. How are Ozone Layer Depletion and climate change related?
Ans: While ozone layer depletion and climate change are distinct issues, they are interconnected. Ozone-depleting substances, such as CFCs, are also potent greenhouse gases that contribute to global warming. Additionally, changes in the ozone layer can affect atmospheric circulation, influencing weather patterns and contributing to climate change.
6. How can individuals help prevent Ozone Layer Depletion?
Ans: Individuals can help to protect the ozone layer by:
- Avoiding products with ODS, such as older appliances that use CFCs.
- Supporting green energy and reducing personal carbon footprints.
- Raising awareness about the importance of ozone layer protection and the harmful effects of ODS.
7. Why is Ozone Layer Depletion dangerous?
Ans: Ozone Layer Depletion is dangerous because it increases the amount of harmful UV-B radiation reaching the Earth’s surface. This can lead to serious health problems, such as skin cancer and cataracts, as well as damage to ecosystems, marine life, crops, and wildlife.




SocialMediaGirls I like the efforts you have put in this, regards for all the great content.
Clochant This was beautiful Admin. Thank you for your reflections.